Abstract
T-lineage acute lymphoblastic leukemia (T-ALL) is considered as a high-risk disease entity that is associated with treatment resistance and relapse, and while there is only a 15% incidence of T-ALL among ALL patients, T-ALL represents 50% of patients that receive high-risk treatment based on residual disease levels during the initial phase of treatment. Glucocorticoids (GC) are cornerstone drugs in the treatment of ALL, and initial GC-responses strongly predict for survival and cure. However, cellular mechanisms of GC-resistance in ALL patients are poorly understood. We recently published our integrated genomics data for 69 primary T-ALL patient samples in relation to clinical outcome and in vitro drug responses (Li et al., PLoS Medicine, 2016).In that study, we identified activating mutations in the IL7R signaling pathway as cause of GC-resistance in T-ALL. Functional modeling of IL7R signaling mutations in two GC-sensitive T-ALL cell lines demonstrated that GC-resistance is not due to an altered transcriptional response by the glucocorticoid receptor NR3C1. We found that IL7R signaling mutations provoke strong MAPK and AKT signaling resulting in up-regulation of anti-apoptotic BCL2 family members and a shift in the molecular size of BIM, an essential pro-apoptotic molecule for GC-induced death. Another independent study linked strong induction of JAK/STAT signaling in response to interleukin-7 (IL7) to GC-resistance in T-ALL patients (Delgado-Martin et al., Leukemia, 2017).
Following these observations, we now focus on improving our understanding on the role of BCL2 family members as effectors of GC-resistance downstream of IL7R signaling mutations and potential strategies to break GC-resistance. We generated inducible T-ALL cell lines that retain a GC-sensitive response following induction of wildtype IL7R or JAK1/3 molecules whereas these lines adopt a GC-resistant phenotype following (induced) expression of mutant IL7R, JAK1 or N/K-RAS molecules or wild-type RAS or AKT. Despite induced GC-resistance, up-regulation of total pro-apoptotic BIM levels is not affected following glucocorticoid exposure. Using specific targeted inhibitors, we found that ERK signaling becomes strongly activated downstream of most (mutant) IL7R-signaling molecules and phosphorylates pro-apoptotic BIM-L and BIM-EL isoforms at multiple residues, which are normally efficiently dephosphorylated by phosphatase PP2A. We currently use immuno-precipitation followed by mass-spectrometry to study the functional consequences of BIM phosphorylation that results in shifts among BCL2 family member interactions and GC-resistance and may point to novel strategies to break GC-resistance.
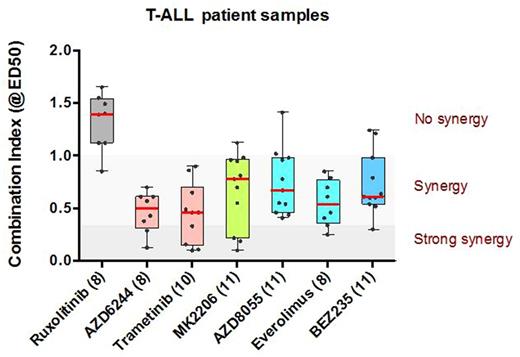
The finding that GC-resistance arises from activated MEK-ERK and AKT suggests that a therapeutic regime combining GC-treatment with MEK, AKT or mTOR inhibitors may increase GC-responsiveness. We tested various compounds including the JAK1 inhibitor ruxolitinib, MEK inhibitors AZD5244 or trametinib, AKT inhibitor MK2206, mTOR inhibitors AZD8055 or everolimus or the dual kinase PI3K-mTOR inhibitor BEZ235 for their abilities to enhance GC-responsiveness of primary T-ALL patient samples using an in-vitro cytotoxicity assay (as measured by the combination index for the effective dose at which 50 percent of the leukemia cells are killed (ED50); see Figure). Despite 3 T-ALL patient samples tested that harbored IL7RA mutations, we found no proof for effectiveness of ruxolitinib to revert GC-resistance. This may possibly be due to a lack of cellular proliferation during in vitro drug sensitivity testing, implying that dormant leukemic stem cells are not killed by ruxolitinib. Both MEK inhibitors AZD6244 or trametinib synergistically enhanced prednisolone responsiveness in 5 out of 9 and 9 out of 10 patient samples, respectively. The AKT inhibitor MK2206 synergistically enhanced prednisolone sensitivity for 8 out of 11 patient samples, while mTOR inhibitors AZD-8055 and everolimus enhanced prednisolone responses in 10 out of 11 and 8 out of 8 patient samples, respectively (including two GC-resistant patients). The PI3K inhibitor NVPBEZ235 synergistically enhanced prednisolone sensitivity in 10 out of 11 patient samples.
In conclusion, our data support the recommendation of including MEK, AKT, mTOR or dual PI3K/mTOR inhibitors to restore-or improve-GC-sensitivity of T-ALL cells to improve outcome.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal